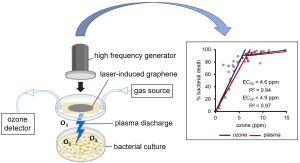
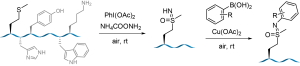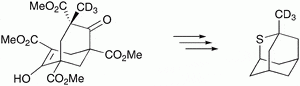86. Larkin, James; Mozden, Sarah; Chyan, Yieu; Zheng, Vivian; Cherukuri, Paul; Tour, James; Ball, Zachary. Capacitively-Coupled Plasma from Laser-Induced Graphene Points to Ozone as the Major Mediator of Antibacterial Activity. ACS Appl. Mater. Interfaces. 2023, 15, 45601-45605. DOI: 10.1021/acsami.3c09216.
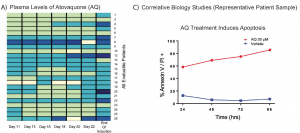
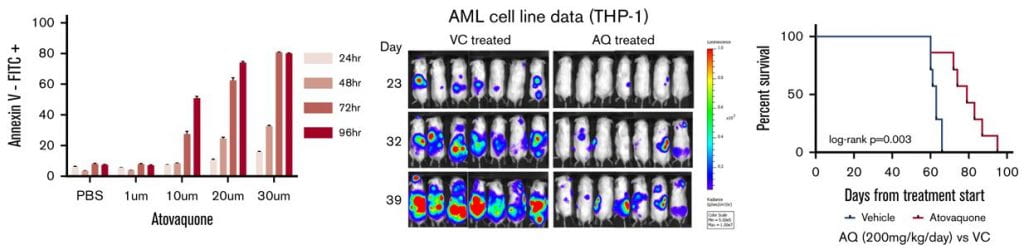
84. Stevens, A. M.; Schafer, E. S.; Li, M.; Terrell, M.; Rashid, R.; Paek, H.; Bernhardt, M. B.; Weisnicht, A.; Smith, W. T.; Keogh, N. J.; Alozie, M. C.; Oviedo, H. H.; Gonzalez, A. K.; Ilangovan, T.; Mangubat-Medina, A.; Wang, H.; Jo, E.; Rabik, C. A.; Bocchini, C.; Hilsenbeck, S.; Ball, Z. T.; Cooper, T. M.; Redell, M. S. Repurposing Atovaquone as a Therapeutic against Acute Myeloid Leukemia (AML): Combination with Conventional Chemotherapy Is Feasible and Well Tolerated. Cancers 2023, 15, 1344. DOI: 10.3390/cancers15041344.

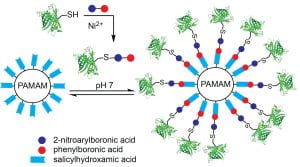
83. Swierczynski, M. J.; Ding, Y.; Ball, Z. T. Dual-Boronic Acid Reagents That Combine Dynamic and Covalent Bioconjugation. Bioconjugate Chem. 2022, 33, 2307–2313. DOI: 10.1021/acs.bioconjchem.2c00508.
82. Ding, Y.; Pedersen, S. S.; Lin, A.; Qian, R.; Ball, Z. T. Direct formation and site-selective elaboration of methionine sulfoximine in polypeptides. Chem. Sci. 2022, 13, 14101–14105. DOI: 10.1039/D2SC04220G.
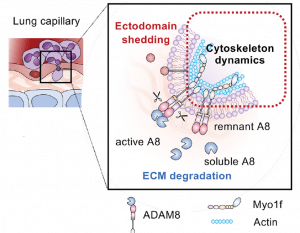
81. Conrad, C.; Yildiz, D.; Cleary, S. J.; Margraf, A.; Cook, L.; Schlomann, U.; Panaretou, B.; Bowser, J. L.; Karmouty-Quintana, H.; Li, J.; Berg, N. K.; Martin, S. C.; Aljohmani, A.; Moussavi-Harami, S. F.; Wang, K. M.; Tian, J. J.; Magnen, M.; Valet, C.; Qiu, L.; Singer, J. P.; Eltzschig, H. K.; Bertrams, W.; Herold, S.; Suttorp, N.; Schmeck, B.; Ball, Z. T.; Zarbock, A.; Looney, M. R.; Bartsch, J. W. ADAM8 signaling drives neutrophil migration and ARDS severity. JCI Insight 2022, 7 DOI: 10.1172/jci.insight.149870.
80. Stevens, A. M.; Schafer, E.; Li, M.; Terrell, M.; Rashid, R.; Paek, H.; Weisnicht, A.; Keogh, N. J.; Alozie, M. C.; Oviedo, H. H.; Gonzalez, A. K.; Mangubat-Medina, A.; Wang, H.; Jo, E.; Rabik, C. A.; Bocchini, C.; Hilsenbeck, S.; Ball, Z. T.; Cooper, T. M.; Redell, M. Combining Atovaquone with Intensive Conventional Chemotherapy for Pediatric Acute Myeloid Leukemia (AML) Is Feasible and Well Tolerated. Blood 2021, 138, 2312. DOI: 10.1182/blood-2021-146057.
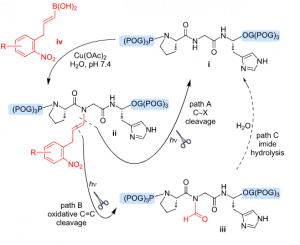
79. Wang, H.; Ball, Z. T. A photochemical C=C cleavage process: toward access to backbone N-formyl peptides. Beilstein J. Org. Chem. 2021, 17, 2932–2938. DOI: 10.3762/bjoc.17.202.
78. Wu, K.-L.; Yu, C.; Lee, C.; Zuo, C.; Ball, Z. T.; Xiao, H. Precision Modification of Native Antibodies. Bioconjugate Chem. 2021, 32, 1947–1959. DOI: 10.1021/acs.bioconjchem.1c00342.
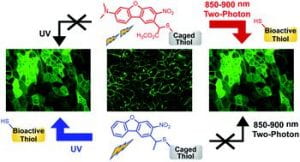
77. Mangubat-Medina, A. E.; Ball, Z. T. Triggering biological processes: methods and applications of photocaged peptides and proteins. Chem. Soc. Rev. 2021, 50, 10403–10421. DOI: 10.1039/D0CS01434F.
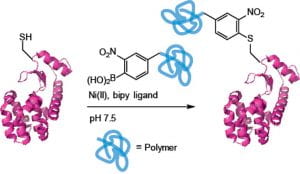
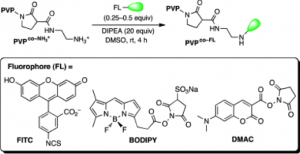
76. Miller, M. K.; Swierczynski, M. J.; Ding, Y.; Ball, Z. T. Boronic Acid Pairs for Sequential Bioconjugation. Org. Lett. 2021, 23, 5334–5338. DOI: 10.1021/acs.orglett.1c01624.
75. Miller, M. K.; Ball, Z. T. Boronic Acid Reagents for Transition-Metal-Mediated Cross-Coupling with Proteins and Peptides. Isr. J. Chem. 2021, 61, 387–393. DOI: 10.1002/ijch.202100012.
74. Hammers, M. D.; Hodny, M. H.; Bader, T. K.; Mahmoodi, M. M.; Fang, S.; Fenton, A. D.; Nurie, K.; Trial, H. O.; Xu, F.; Healy, A. T.; Ball, Z. T.; Blank, D. A.; Distefano, M. D. Two-photon uncaging of bioactive thiols in live cells at wavelengths above 800 nm. Org. Biomol. Chem. 2021, 19, 2213–2223. DOI: 10.1039/D0OB01986K.
73. One-Step Protein–Polymer Conjugates from Boronic-Acid-Functionalized Polymers. Swierczynski, M. J.; Ball, Z. T. Bioconjugate Chem. 2020, 31, 2494–2498. DOI: 10.1021/acs.bioconjchem.0c00516.
72. Copper-mediated peptide arylation selective for the N-terminus. Miller, M. K.; Wang, H.; Hanaya, K.; Zhang, O.; Berlaga, A.; Ball, Z. T. Chem. Sci. 2020, 11, 10501–10505. DOI: 10.1039/D0SC02933E
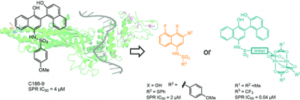
67. Atovaquone is active against AML by upregulating the integrated stress pathway and suppressing oxidative phosphorylation. Stevens, A. M.; Xiang, M.; Heppler, L. N.; Tošić, I.; Jiang, K.; Munoz, J. O.; Gaikwad, A. S.; Horton, T. M.; Long, X.; Narayanan, P.; et al. Blood Adv 2019, 3, 4215–4227.
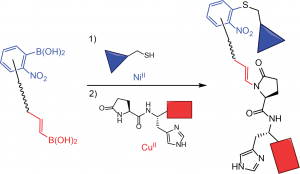
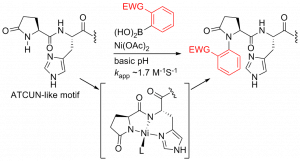
66. Nickel(II)-Promoted N-H Arylation of Pyroglutamate-Histidine with Arylboronic Acid Reagents. Hanaya, K., Miller, M. K., Ball, Z. T. Org. Lett. 2019, 21, 2445-2448.
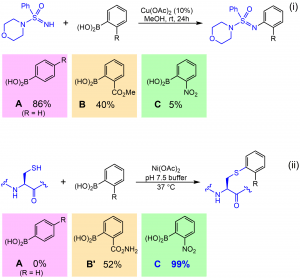
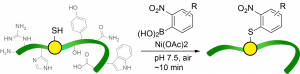
65. Rapid Nickel(II)-Promoted Cysteine S-Arylation with Arylboronic Acids. Hanaya, K., Ohata, J., Miller, M. K., Mangubat-Medina, A. E., Swierczynski, M. J., Yang, D., Rosenthal, R. M., Popp, B., Ball, Z. T. Chem. Commun., 2019, 55, 2841-2844.
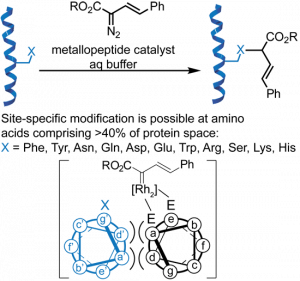
64. Protein Substrates for Reaction Discovery: Site-Selective Modification with Boronic Acid Reagents. Ball, Z. T. Acc. Chem. Res., 2019, 52, 566–575.
63. Metal-Mediated Functionalization of Natural Peptides and Proteins: Panning for Bioconjugation Gold. Ohata, J., Martin, S. C., Ball, Z. T. Angew. Chem. Int. Ed., 2019, 58, 6176-6199.
62. Rhodium at the Chemistry-Biology Interface. Ohata, J., Ball, Z.T. Dalton Trans., 2018, 47, 14855-14860.
61. A Vinylogous Photocleavage Strategy Allows Direct Photocaging of Backbone Amide Structure. Mangubat-Medina, A. E., Martin, S. C., Hanaya, K., Ball, Z.T. J. Am. Chem. Soc., 2018, 140, 8401–8404.
60. A Naturally Encoded Dipeptide Handle for Bioorthogonal Chan-Lam Coupling. Ohata, J., Zeng, Y., Segatori, L., Ball, Z.T. Angew. Chem. Int. Ed., 2018, 57, 4015–4019.
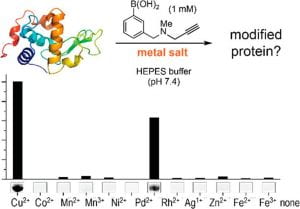
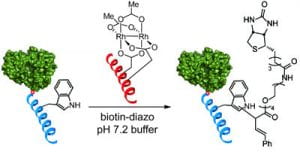
59. A Three-Component Organometallic Tyrosine Bioconjugation. Ohata, J., Miller, M.K., Mountain, C., Ball, Z.T. Angew. Chem. Int. Ed., 2018, 57, 2827–2830.
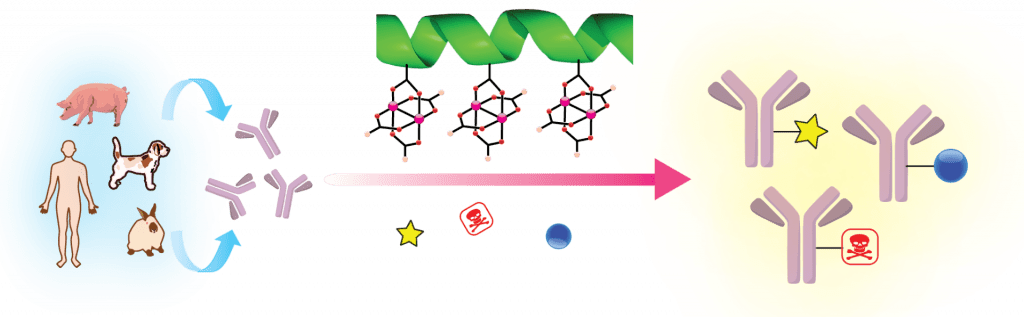
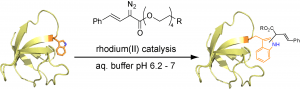
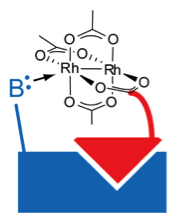
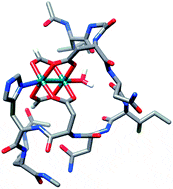
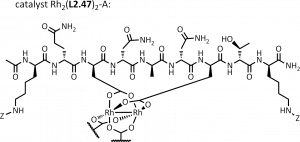
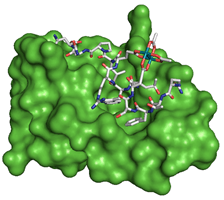
58. A Hexa-Rhodium Metallopeptide Catalyst for Site-Specific Functionalization of Natural Antibodies. Ohata, J., Ball, Z.T. J. Am. Chem. Soc., 2017, 139, 12617–12622.
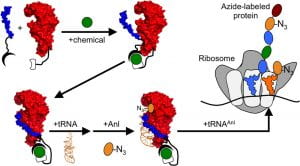
57. Programming Post-Translational Control over the Metabolic Labeling of Cellular Proteins with a Noncanonical Amino Acid. Thomas, E.; Pandey, N.; Knudsen, S.; Ball, Z.T.; Silberg, J. ACS Synth. Biol. , 2017, 6, 1572–1583.
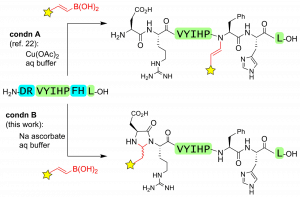
56. Ascorbate as a Pro-Oxidant: Mild N-Terminal Modification with Vinylboronic Acids. Ohata, J., Ball, Z.T. Chem. Commun., 2016, 53, 1622–1625.
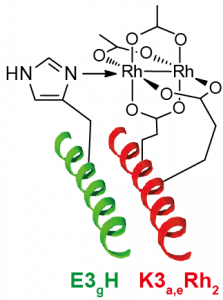
55. Designing Selectivity in Dirhodium Metallopeptide Catalysts for Protein Modification. Martin, S.C.; Vohidov, F.; Wang, H.; Knudsen, S.; Marzec, A.A.; Ball, Z.T. Bioconj. Chem., 2016, 28, 659–665.
54. Chemical Posttranslational Modification with Designed Rhodium(II) Catalysts. Martin, S.C., Minus, M.B.; Ball, Z.T. In Methods in Enzymology, Vol. 580. Pecoraro, V., Ed.; Academic Press: 2016; 1-19.
53. Assessing the intracellular fate of rhodium(II) complexes. Minus, M.B., Kang, M.K., Knudsen, S.E.; Liu, W., Krueger, M.J.; Redell, M.S.; Ball, Z.T. Chem. Commun., 2016, 52, 11685–11688.
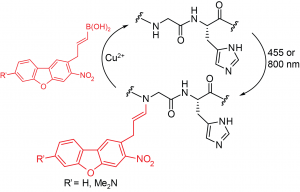
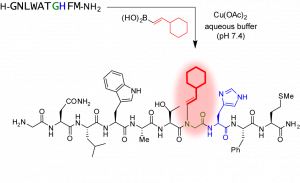
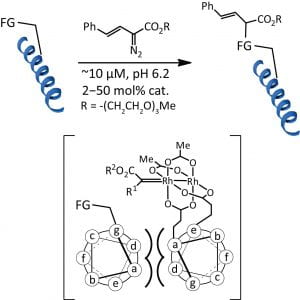
52. Histidine-Directed Arylation/Alkenylation of Backbone N-H Bonds Mediated by Copper(II). Ohata, J.; Minus, M.B.; Abernathy, M.E.; Ball, Z.T. J. Am. Chem. Soc., 2016, 138, 7472-7475.
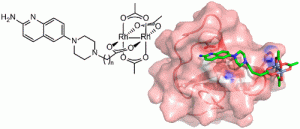
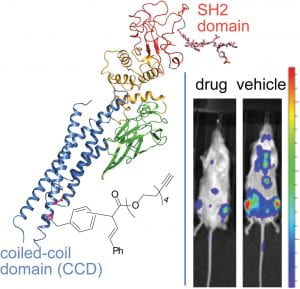
51. Rhodium(II) Proximity-Labeling Identifies a Novel Target Site on STAT3 for Inhibitors with Potent Anti-Leukemia Activity. Minus, M.B.; Liu, W.; Vohidov, F.; Kasembeli, M.M.; Long, X.; Krueger, M.; Stevens, A.; Kolosov, M.I.; Tweardy, D.J.; Redell, M.S.; Ball, Z.T. Angew. Chem. Int. Ed., 2015, 54, 13085-13089.
50. Designing Enzyme-Like Catalysts: A Rhodium(II) Metallopeptide Case Study. Vohidov, F.; Popp, B.V.; Ball, Z.T. In Proceedings of the 24th American Peptide Symposium. Orlando, June 20-25, 2015; Srivastava, V., Yudin, A., Lebl, M., Eds.; American Peptide Society: 2015; 24-26.
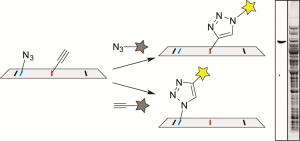
49. Convenient Analysis of Protein Modification by Chemical Blotting with Fluorogenic “Click” Reagents.
Ohata, J.; Vohidov, F.; Ball, Z.T. Mol. Biosyst., 2015, 11, 2846-2849.
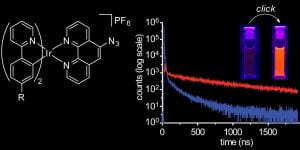
48. Luminogenic Iridium Azide Complexes. Ohata, J.; Vohidov, F.; Aliyan, A.; Huang, K.; Marti, A.A.; Ball, Z.T. Chem. Commun., 2015, 51, 15192-15195.
47. Potent and Selective Inhibition of SH3 Domains with Dirhodium Metalloinhibitors. Vohidov, F.; Knudsen, S.E.; Leonard, P.G.; Ohata, J.; Wheadon, M.J.; Popp, B.V.; Ladbury, J.E.; Ball, Z.T. Chem. Sci., 2015, 6, 4778-4783.
46. Molecular Recognition in Protein Modification with Rhodium Metallopeptides. Ball, Z.T. Curr. Opin. Chem. Biol., 2015, 25, 98-102.
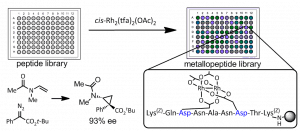
45. Metallopeptide Catalyst Design Enables Fine Control in Selective Functionalization of Natural SH3 Domains. Vohidov, F.; Coughlin, J. M.; Ball, Z.T. Angew. Chem. Intl. Ed., 2015, 54, 4587-4591.
44. Inhibiting Prolyl Isomerase Activity by Hybrid Organic-Inorganic Molecules Containing Rhodium(II) Fragments. Coughlin, J. M.; Kundu, R.; Cooper J.C.; Ball, Z.T. Bioorg. Med. Chem. Lett., 2014, 24, 5203-5206.
43. Stabilization and Functionalization of Single-Walled Carbon Nanotubes with Polyvinylpyrrolidone Copolymers for Applications in Aqueous Media.
Popp, B. V.; Miles, D. H.; Smith, J. A.; Fong, I. M.; Pasquali, M.; Ball, Z. T. J. Polym. Sci. Part A Polym. Chem., 2014, 53, 337-343.
42. Mixed Bioengineering-Chemical Synthesis Approach for the Efficient Preparation of Δ7-Dafachronic Acid. Kinzurik, M.I.; Hristov, L.V.; Matsuda, S.P.T.; Ball, Z.T. Org. Lett., 2014, 16, 2188-2191.
41. A Tripodal Peptide Ligand for Asymmetric Rh(II) Catalysis Highlights Unique Features of On-Bead Catalyst Development. Sambasivan, R.; Zheng, W.; Burya, S.J.; Popp, B.V.; Turro, C.; Clementi, C.; Ball, Z.T. Chem. Sci., 2014, 5, 1401-1407.
40. Studies of Asymmetric Styrene Cyclopropanation with a Rhodium(II) Metallopeptide Catalyst Developed with a High-Throughput Screen.
Sambasivan, R.; Ball, Z.T. Chirality, 2013, 25, 493-497.
39. A Rhodium-Catalyzed Method for Serum-Stable Cysteine Modification. Kundu, R.; Ball, Z.T. ChemComm, 2013, 49, 4166-4168.
38. Designing Enzyme-Like Catalysts: A Metallopeptide Case Study. Ball, Z.T. Accts. Chem. Res., 2013, 46, 560-570.
37. Determination of Orientational Isomerism in Rhodium(II) Metallopeptides by Pyrene Fluorescence. Sambasivan, R.; Ball, Z.T. Org. Biomol. Chem., 2012, 10, 8203-8206.
36. Screening Rhodium Metallopeptide Libraries on Bead: Asymmetric Cyclopropanation and a Solution to the Enantiomer Problem.
Sambasivan, R.; Ball, Z.T. Angew. Chem., Int. Ed., 2012, 51, 8568-8572.
35. Sequence-Specific Inhibition of a Designed Metallopeptide Catalyst. Popp, P.V.; Chen, Z.; Ball, Z. ChemComm , 2012, 48, 7492-7494.
34. Hybrid Organic-Inorganic Inhibitors of a PDZ Interaction that Regulates the Endocytic Fate of CFTR.
Kundu, R.; Cushing, P.R.; Popp, B.V.; Zhao, Y; Madden, D.R.; Ball, Z.T. Angew. Chem., Int. Ed., 2012, 51, 7217-7220.
33. Catalytic Protein Modification with Dirhodium Metallopeptides: Specificity in Designed and Natural Systems.
Chen, Z.; Vohidov, F.; Coughlin, J.M.; Stagg, L.J.; Arold, S.T.; Ladbury, J.E.; Ball, Z.T. J. Am. Chem. Soc., 2012, 134, 10138-10145.
32. Organometallics Roundtable 2011. Gladysz, J.A.; Ball, Z.T.; Bertrand, G, Blum, S.A.; Dong, V.M.; Dorta, R; Hahn, F.E. Humphrey, M.G.; Jones, W.D.; Klosin, J.; Manners, I.; Marks, T.J.; Mayer, J.M.; Rieger, B. Ritter, J.C.; Sattelberger, A.P.; Schomaker, J.M.; and Yam V.W. Organometallics, 2012, 1-18
31. A General Synthesis of Dirhodium Metallopeptides as MDM2 Ligands.
Zaykov, A.N.; Ball, Z.T. Chem. Commun., 2011, 47, 10927-10929.
30. Site-Specific Protein Modification with a Dirhodium Metallopeptide Catalyst. Chen, Z.; Popp, B. V.; Bovet, C. L.; Ball, Z. T. ACS Chem. Biol., 2011, 6, 920–925.
29. Kinetic and Stereoselectivity Effects of Phosphite Ligands in Dirhodium Catalysis. Zaykov, A.N.; Ball, Z.T. Tetrahedron, 2011, 67, 4397–4401. Tetrahedron Young Investigator Award 2011: F. Dean Toste.
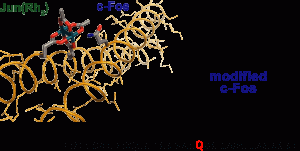
28. Proximity-Driven Metallopeptide Catalysis: Remarkable Side-Chain Scope Enables Modification of the Fos bZip Domain. Popp, B.V.; Ball, Z.T. Chem. Sci., 2011, 2, 690–695.
27. Metallopeptides for Asymmetric Dirhodium Catalysis.
Sambasivan, R.; Ball, Z.T. J. Am. Chem. Soc., 2010, 132, 9289–9291.
26. Copper-Catalyzed Remote sp3 C–H Chlorination of Alkyl Hydroperoxides.
Kundu, R.; Ball, Z.T. Org. Lett., 2010, 12, 2460–2463.
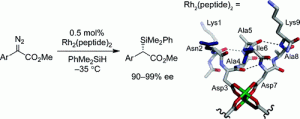
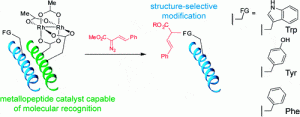
25. Structure-Selective Modification of Aromatic Side Chains with Dirhodium Metallopeptide Catalysts. Popp, B.V.; Ball, Z.T. J. Am. Chem. Soc., 2010, 132, 6660–6662.
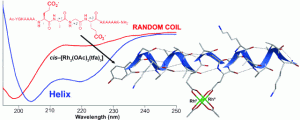
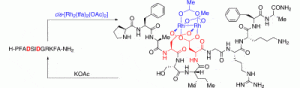
24. Helix Induction by Dirhodium: Access to Biocompatible Metallopeptides with Defined Secondary Structure. Zaykov, A.N.; Popp, B.V.; Ball, Z.T. Chem. Eur. J., 2010, 16, 6651–6659.
23. Allylcopper Intermediates with N-Heterocyclic Carbene Ligands: Synthesis, Structure, and Catalysis. Russo, V.; Herron, J.R.; Ball, Z.T. Org. Lett., 2010, 12, 220–223.
22. Controlling Peptide Structure with Coordination Chemistry: Robust and Reversible Peptide-Dirhodium Ligation. Zaykov, A.N.; MacKenzie, K.R.; Ball, Z.T. Chem. Eur. J. 2009, 15, 8961–8965.
21. Asymmetric Total Synthesis of Soraphen A: A Flexible Alkyne Strategy. Trost, B.M.; Sieber, J.D.; Qian, W.; Dhawan, R.; Ball, Z.T. Angew. Chem., Int. Ed., 2009, 48, 5478–5481.
20. Catalytic Organocopper Chemistry from Organosiloxane Reagents. Herron, J.R.; Russo, V.; Valente, E.J.; Ball, Z.T. Chem. Eur. J.. 2009, 15, 8713–8716.
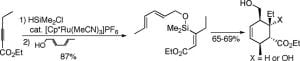
19. Synthesis and Isotopic Labeling of a Naturally Occurring Alkyl-Thiadiamondoid. Russo, V.; Allen, J.; Ball, Z.T. Chem. Commun. 2009, 30, 595–596.
18. Synthesis and Reactivity of Functionalized Arylcopper Compounds by Transmetalation of Organosilanes. Herron, J.R.; Ball, Z.T. J. Am. Chem. Soc.2008, 130, 16486–16487.
17. Hydrosilylation of Alkynes and Related Reactions. Ball, Z.T. in Comprehensive Organometallic Chemistry, 3rd ed.; Mingos, M.; Crabtree, R., Eds. Elsevier Ltd.: London, 2007; Vol. 10, pp 789–814.
16. Amphiphilic Diblock Copolymer Compatibilizers and Their Effect on the Morphology and Performance of P3HT:PCBM Solar Cells. Sivula, K.; Ball, Z.T.; Watanabe, N.; Fréchet, J.M.J. Adv. Mater. 2006, 18, 206.
15. Well-Defined, Living Polymers with High Fullerene Content and Their Use in Block Copolymers for Solution-Phase and Bulk Organization. Ball, Z.T.; Sivula, K.; Fréchet, J.M.J.Macromolecules 2006, 39, 70–72.
14. Alkyne Hydrosilylation Catalyzed by a Cationic Ruthenium Complex: Efficient and General Trans Addition. Trost, B.M.; Ball, Z.T. J. Am. Chem. Soc.2005, 127, 17644–17655.
13. Selective Synthesis of Functionalized, Tertiary Silanes by Diastereoselective Rearrangement-Addition. Trost, B.M.; Ball, Z.T.; Kang, E.-J. Org. Lett. 2005,7, 4911–4913.
12. An Alkyne Hydrosilylation-Oxidation Strategy for the Selective Installation of Oxygen Functionality. Trost, B.M.; Ball, Z.T.; Laemmerhold, K. M. J. Am. Chem. Soc. 2005, 127, 10028–10038.
11. Addition of Metalloid Hydrides to Alkynes: Hydrometallation with Boron, Silicon, and Tin. Trost, B.M.; Ball, Z.T. Synthesis 2005, 853–887.
10. Synthetic Stitching with Silicon: Geminal Alkylation-Hydroxylation of Alkynyl Carbonyl Compounds. Trost, B.M.; Ball, Z.T. J. Am. Chem. Soc. 2004,126, 13942–13944.
9. A Theoretical Study on the Mechanism, Regiochemistry, and Stereochemistry of Hydrosilylation Catalyzed by Cationic Ruthenium Complexes. Chung, L. W.; Wu, Y.-D.; Trost, B.M.; Ball, Z. T. J. Am. Chem. Soc. 2003, 125, 11578–11582.
8. Regioselective Hydrosilylation of Propargylic Alcohols: An Aldol Surrogate. Trost, B. M.; Ball, Z. T.; Jöge, T. Angew. Chem., Int. Ed. 2003, 42, 3415–3418.
7. Ruthenium-Catalyzed Vinylsilane Synthesis and Cross-Coupling as a Selective Approach to Alkenes: Benzyldimethylsilyl as a Robust Vinylmetal Functionality. Trost, B.M.; Machacek, M. R.; Ball, Z.T. Org. Lett. 2003, 5, 1895–1898.
6. Intramolecular Endo-Dig Hydrosilylation Catalyzed by Ruthenium: Evidence for a New Mechanistic Pathway. Trost, B.M.; Ball, Z.T. J. Am. Chem. Soc.2003, 125, 30–31.
5. A Stereospecific Ruthenium-Catalyzed Allylic Alkylation. Trost, B.M.; Fraisse, P.L.; Ball, Z.T. Angew. Chem., Int. Ed. 2002, 41, 1059–1061.
4. A Chemoselective Reduction of Alkynes to (E)-Alkenes. Trost, B.M.; Ball, Z.T.; Jöge, T. J. Am. Chem. Soc. 2002, 124, 7922–7923.
3. Markovnikov Alkyne Hydrosilylation Catalyzed by Ruthenium Complexes. Trost, B.M.; Ball, Z.T. J. Am. Chem. Soc. 2001, 123, 12726–12727.
2. A Synthetic Library of Cell-Permeable Molecules. Koide, K.; Finkelstein, J.M.; Ball, Z.; Verdine, G.L. J. Am. Chem. Soc. 2001, 123, 398–408.
1. Design and Synthesis of Novel NK1/NK2 Dual Antagonists. Reichard, G.A.; Ball, Z.T.; Aslanian, R.; Anthes, J.C.; Shih, N.Y.; Piwinski, J.J. Bioorg. Med. Chem. Lett. 2000, 10, 2329–2332.